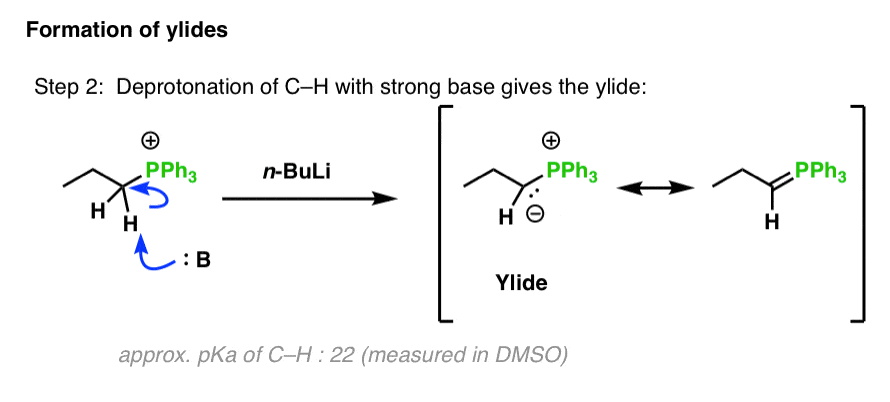
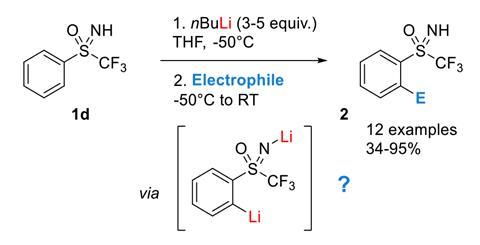
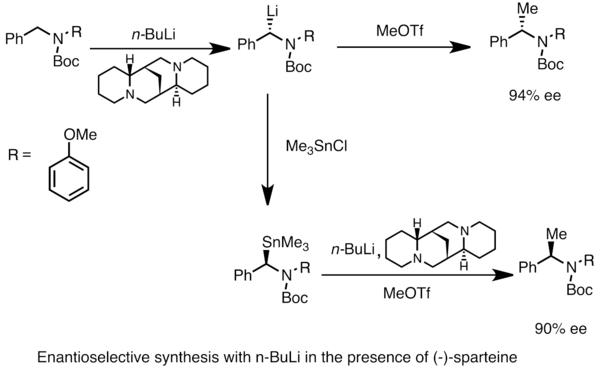
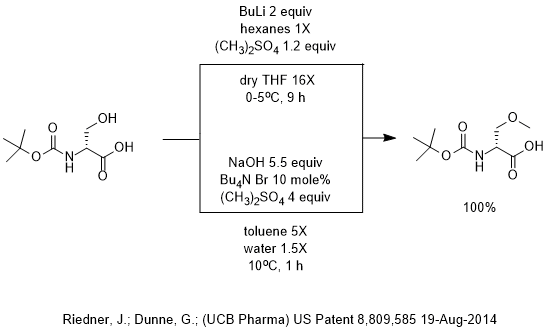
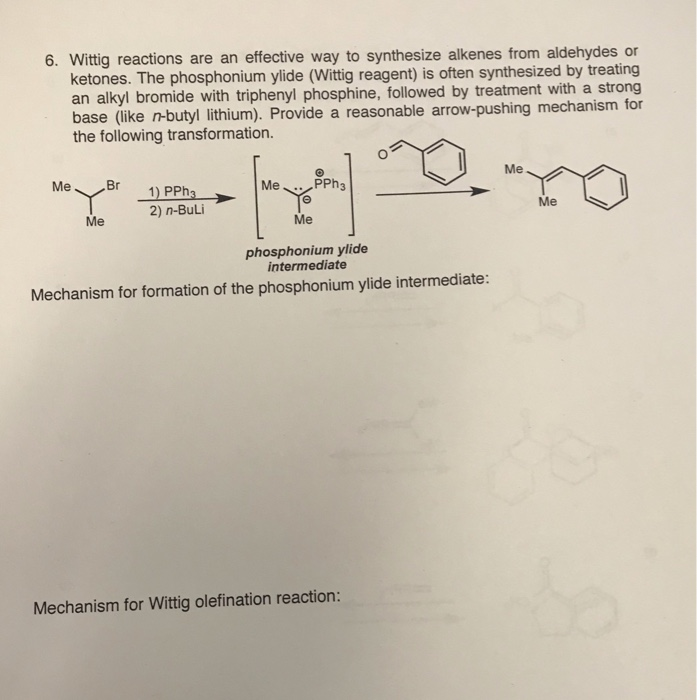
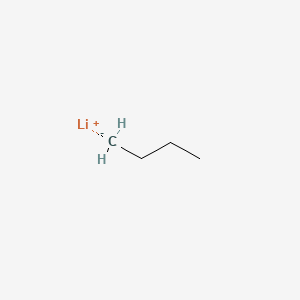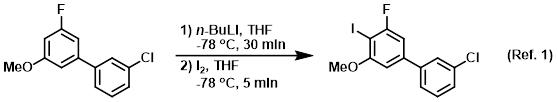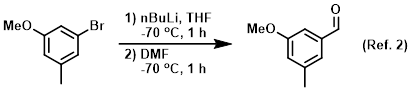n‐Butyllithium (1 mol %)‐catalyzed Hydroboration of Aldehydes and Ketones with Pinacolborane - Yang - 2019 - Bulletin of the Korean Chemical Society - Wiley Online Library

Mechanism of the Deprotonation Reaction of Alkyl Benzyl Ethers with n‐ Butyllithium - Raposo - 2013 - Chemistry – A European Journal - Wiley Online Library

n-BuLi/LiCH2CN-Mediated One-Carbon Homologation of Aryl Epoxides into Conjugated Allyl Alcohols | Organic Letters

Mechanism of the Deprotonation Reaction of Alkyl Benzyl Ethers with n‐ Butyllithium - Raposo - 2013 - Chemistry – A European Journal - Wiley Online Library

Grignard Reaction Key features: Handling of air/ moisture sensitive chemicals, formation of C-C bond. n-Butyl lithium Key features: Strong base such as. - ppt download

n-Butyllithium, 1.6M solution in hexanes, AcroSeal , Thermo Scientific Chemicals, Quantity: 100 mL | Fisher Scientific